- Infectious Diseases of Livestock
- Part 2
- Bovine respiratory syncytial virus infection
- GENERAL INTRODUCTION: PARAMYXOVIRIDAE AND PNEUMOVIRIDAE
- Rinderpest
- Peste des petits ruminants
- Parainfluenza type 3 infection
- Bovine respiratory syncytial virus infection
- Hendra virus infection
- Paramyxovirus-induced reproductive failure and congenital defects in pigs
- Nipah virus disease
- GENERAL INTRODUCTION: CALICIVIRIDAE AND ASTROVIRIDAE
- Vesicular exanthema
- Enteric caliciviruses of pigs and cattle
- GENERAL INTRODUCTION: RETROVIRIDAE
- Enzootic bovine leukosis
- Jaagsiekte
- Visna-maedi
- Caprine arthritis-encephalitis
- Equine infectious anaemia
- GENERAL INTRODUCTION: PAPILLOMAVIRIDAE
- Papillomavirus infection of ruminants
- Papillomavirus infection of equids
- GENERAL INTRODUCTION: ORTHOMYXOVIRIDAE
- Equine influenza
- Swine influenza
- GENERAL INTRODUCTION: CORONAVIRIDAE
- Porcine transmissible gastroenteritis
- Porcine respiratory coronavirus infection
- Porcine epidemic diarrhoea
- Porcine haemagglutinating encephalomyelitis virus infection
- Porcine deltacoronavirus infection
- Bovine coronavirus infection
- Ovine coronavirus infection
- Equine coronavirus infection
- GENERAL INTRODUCTION: PARVOVIRIDAE
- Porcine parvovirus infection
- Bovine parvovirus infection
- GENERAL INTRODUCTION: ADENOVIRIDAE
- Adenovirus infections
- GENERAL INTRODUCTION: HERPESVIRIDAE
- Equid herpesvirus 1 and equid herpesvirus 4 infections
- Equid gammaherpesvirus 2 and equid gammaherpesvirus 5 infections
- Equine coital exanthema
- Infectious bovine rhinotracheitis/infectious pustular vulvovaginitis and infectious pustular balanoposthitis
- Bovine alphaherpesvirus 2 infections
- Malignant catarrhal fever
- Pseudorabies
- Suid herpesvirus 2 infection
- GENERAL INTRODUCTION: ARTERIVIRIDAE
- Equine viral arteritis
- Porcine reproductive and respiratory syndrome
- GENERAL INTRODUCTION: FLAVIVIRIDAE
- Bovine viral diarrhoea and mucosal disease
- Border disease
- Hog cholera
- Wesselsbron disease
- Louping ill
- West nile virus infection
- GENERAL INTRODUCTION: TOGAVIRIDAE
- Equine encephalitides caused by alphaviruses in the Western Hemisphere
- Old World alphavirus infections in animals
- Getah virus infection
- GENERAL INTRODUCTION: BUNYAVIRIDAE
- Diseases caused by Akabane and related Simbu-group viruses
- Rift Valley fever
- Nairobi sheep disease
- Crimean-Congo haemorrhagic fever
- GENERAL INTRODUCTION: ASFARVIRIDAE
- African swine fever
- GENERAL INTRODUCTION: RHABDOVIRIDAE
- Rabies
- Bovine ephemeral fever
- Vesicular stomatitis and other vesiculovirus infections
- GENERAL INTRODUCTION: REOVIRIDAE
- Bluetongue
- Ibaraki disease in cattle
- Epizootic haemorrhagic disease
- African horse sickness
- Equine encephalosis
- Palyam serogroup orbivirus infections
- Rotavirus infections
- GENERAL INTRODUCTION: POXVIRIDAE
- Lumpy skin disease
- Sheeppox and goatpox
- Orf
- Ulcerative dermatosis
- Bovine papular stomatitis
- Pseudocowpox
- Swinepox
- Cowpox
- Horsepox
- Camelpox
- Buffalopox
- GENERAL INTRODUCTION: PICORNAVIRIDAE
- Teschen, Talfan and reproductive diseases caused by porcine enteroviruses
- Encephalomyocarditis virus infection
- Swine vesicular disease
- Equine picornavirus infection
- Bovine rhinovirus infection
- Foot-and-mouth disease
- GENERAL INTRODUCTION: BORNAVIRIDAE
- Borna disease
- GENERAL INTRODUCTION: CIRCOVIRIDAE AND ANELLOVIRIDAE
- Post-weaning multi-systemic wasting syndrome in swine
- GENERAL INTRODUCTION: PRION DISEASES
- Scrapie
- Bovine spongiform encephalopathy
- Transmissible spongiform encephalopathies related to bovine spongiform encephalopathy in other domestic and captive wild species
Bovine respiratory syncytial virus infection
This content is distributed under the following licence: Attribution-NonCommercial CC BY-NC  View Creative Commons Licence details here
View Creative Commons Licence details here
Bovine respiratory syncytial virus infection
Previous authors: M VAN VUUREN
Current authors:
J L MCGILL - Assistant Professor, MS, PhD, Department of Veterinary Microbiology and Preventive Medicine, Iowa State University, 1907 Christensen Drive, VMRI Building 5, Iowa, 50011, United States of America
RE SACCO - Research Microbiologist/Immunologist /Lead Scientist, PhD, Ruminant Diseases and Immunology Research Unit, National Animal Disease Center/USDA/ARS, 1920 Dayton Avenue, Ames, Iowa, IA 50010, United States of America
Introduction
Infection with bovine respiratory syncytial virus (BRSV) is inapparent in the majority of animals, but in some it does cause mild to severe23 respiratory tract disease characterized by fever, coughing, serous nasal and ocular discharges, dyspnoea and, in some animals, subcutaneous emphysema. It is one of several viruses that are primary pathogens in the bovine respiratory disease complex (see Pneumonic pasteurellosis in cattle). Respiratory syncytial virus also infects sheep and goats and may cause rhinitis in sheep.25 Evidence for the existence of a respiratory syncytial virus (RSV) affecting horses has been reported.24
The virus was first isolated from cattle in Switzerland in 197040 and has subsequently been found worldwide. Its prevalence and importance in Africa is largely unknown, although antibody to it is widespread in feedlot cattle in South Africa.61
Several reviews on BRSV have been published.3, 7, 28, 36, 44, 46, 59
Aetiology
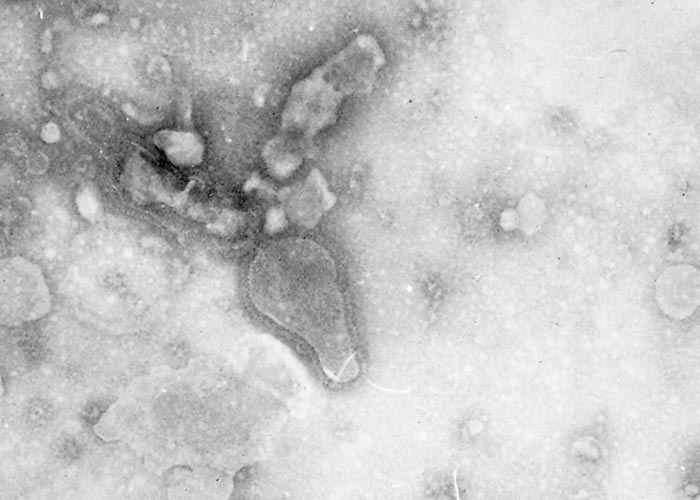
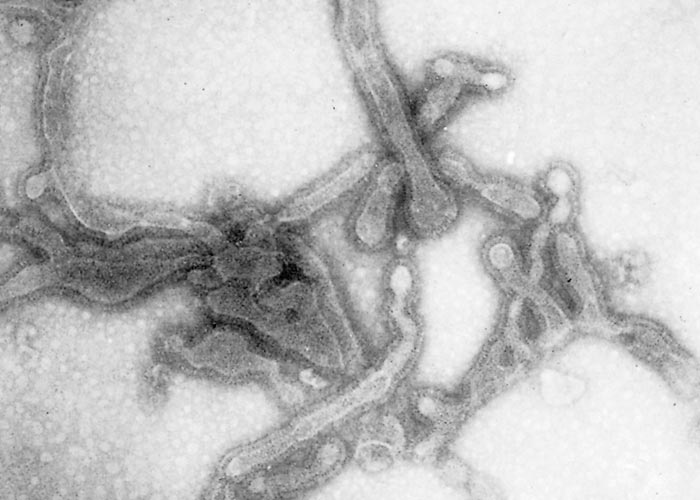
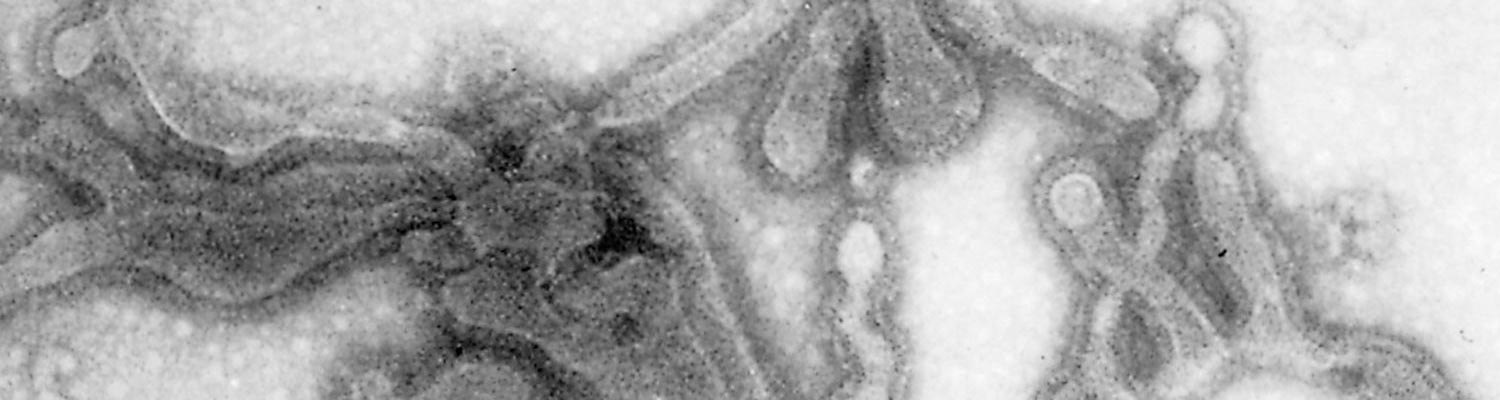
The virus is classified as a member of the genus Pneumovirus in the family Paramyxoviridae. Virions are pleomorphic, enveloped and measure 80 to 500 nm in diameter for the round and pleomorphic forms (Figure 1), and up to 5 microns in length for filamentous forms (Figure 2). The elongated nucleocapsid is helically coiled and tortuous and contains single-stranded RNA.
The envelope contains transmembrane surface glyoproteins, but, in contrast to ortho- and paramyxoviruses, pneumoviruses lack neuraminidase and haemagglutinin. Virions mature by budding from the cytoplasmic membrane.
A wide range of cell cultures are susceptible to infection with BRSV, including those prepared from most bovine tissue types, as well as kidney cells of pigs, hamsters, monkeys and humans.38 Cytopathic effects are characterized by multinucleate syncytial cell formation, intracytoplasmic inclusions and necrosis.
Bovine respiratory syncytial virus is antigenically related to human respiratory syncytial virus (HRSV),40 but the two viruses are distinct.48 It has been proposed that ruminant RSVs can be divided into 2 subgroups, BRSV and ovine RSV, with caprine RSV more closely related to BRSV.20
The genome of respiratory syncytial viruses is approximately 15.2 kb and is transcribed into 10 major subgenomic mRNAs encoding 11 proteins, because M2 mRNA contains two overlapping reading frames, which encode 2 proteins, M2-1 and M2-2.16
Epidemiology
Cattle are probably the principal reservoirs of the virus. Sheep and goats may also be infected but their role in the epidemiology is not clear, and inapparent infections are common in these species.2, 15, 19, 20, 32, 37, 43
Since the initial isolation of BRSV in Switzerland in 1970, the virus has been detected worldwide in cattle herds, with rates of infection impacted by multiple factors. In some countries, it has been estimated that frequency of BRSV exposure exceeds 50 per cent in some dairy and beef herds.28 Respiratory disease due to BRSV is most severe in calves less than 6 months of age, and infection can occur in the presence of maternal antibody. In some geographic areas, by 12 months of age, exposure of calves to BRSV may exceed 70 per cent.58 Reinfection in calves is common, likely as a result of reintroduction of BRSV into herds.36 Spread of the infection may result from direct contact with or aerosol droplets from infected animals, or indirectly from contaminated surfaces.29 Respiratory disease in cattle in the northern hemisphere caused by BRSV occurs mainly in late autumn, winter and early spring, although outbreaks have also been reported in the summer. In addition to a role for fluctuations in temperature, risk of BRSV infections can be impacted by stresses associated with weaning, transport, handling, crowding, and mixing of cattle from different sources.
Pathogenesis
The severity of clinical disease caused by BRSV is dependent on the age and immune status of the calf, the route of infection, and the strain of the virus.8 Some strains are significant primary pathogens of young cattle because, acting alone, they are capable of causing severe damage to the lower respiratory tract.12, 39, 45 However, the virus more frequently predisposes the respiratory tract of cattle to the pathogenic effects of secondary bacterial infections.6
The virus replicates in epithelial cells of the nasal cavity, pharynx, trachea, bronchi and bronchioli, and in type II pneumocytes and alveolar macrophages. It induces cytopathic changes characterized by loss of cilia and/or necrosis of bronchial and bronchiolar epithelial cells.11 The virus also alters opsonization and phagocytosis by alveolar macrophages.38, 56 Reduced mucociliary clearance resulting from a loss of cilia is...
To see the full item, register today: